PLM Tools
PLM Requirements Specification
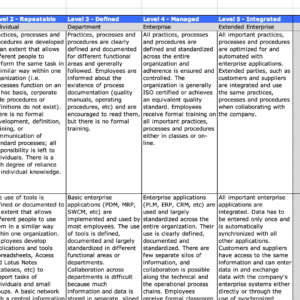
PLM Best Practice Process Maps
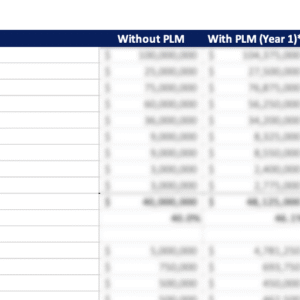
PLM Implementation Cost Estimator
PLM Value and Benefits Calculator
PLM Evaluation Project Plan
PLM Implementation Project Plan
PLM OCM Plan
PLM Requirements Specification
Defining a PLM specification with detailed, business relevant, selective and measurable requirements is critical to ensure the selection of the best PLM solution for your business needs.
Our electronic PLM requirements specification is a key element in enabling you to define a PLM solution that is tailored exactly to your company’s needs. Depending on the situation, you can leverage this tool in a variety of ways:
- The definition of detailed, customized PLM requirements specification as part of a request for information (RFI) or request for proposal (RFP)
- The electronic pre-selection of appropriate PLM solutions
- As a questionnaire for PLM software vendors
- For PLM gap analyses
- For PLM software validation, specifically in the medical device industry. The General Principles of Software Validation; Final Guidance for Industry and FDA staff; Section 4.1 states that “A documented software requirements specification provides a baseline for both validation and verification. The software validation process cannot be completed without an established software requirements specification (Ref: 21 CFR 820.3(z) and (aa) and 820.30(f) and (g))”.
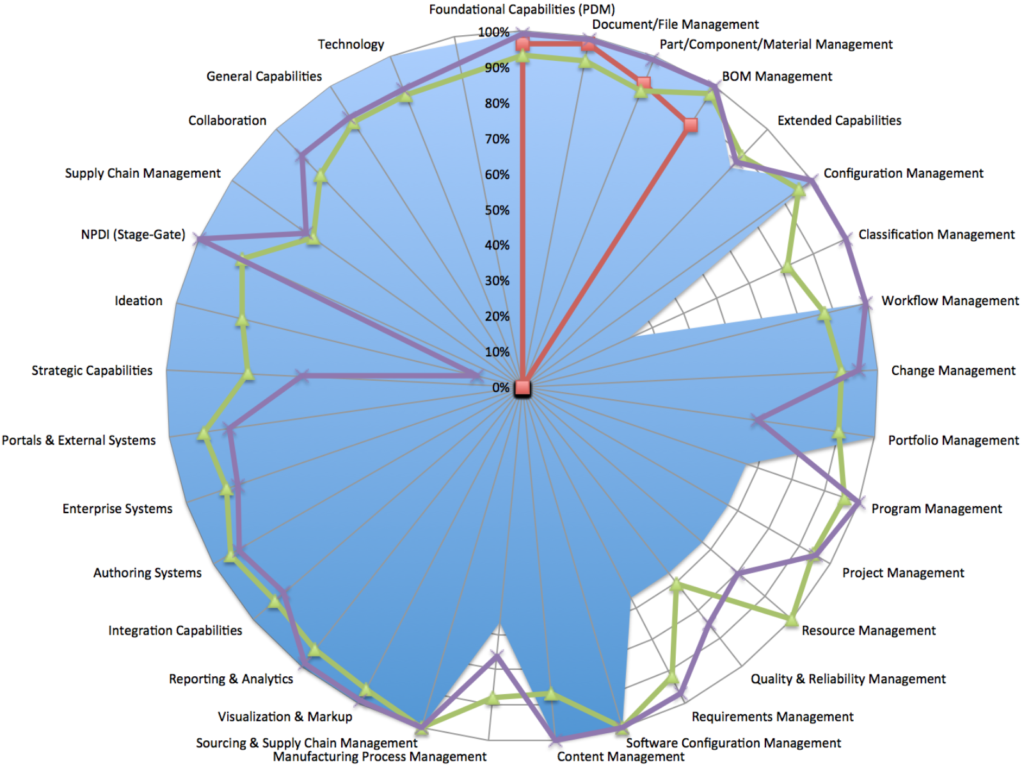
Our newest electronic PLM Requirements Specification includes over 1,200 standard requirements in 37 major functional PLM areas and 75 sub-categories, which according to our experience typically cover over 90% of the PLM needs of most companies. The remaining 10% can be defined based your specific and unique business needs and easily added to the specification.
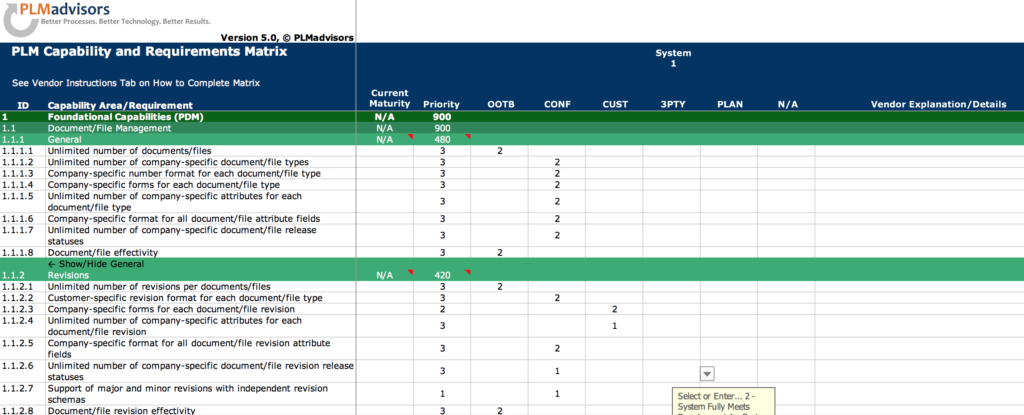
Detailed technical and functional PLM requirements are defined for the following major areas:
- Document/File Management
- Part/Component/Material/Ingredient Management
- BOM/Formulation/Recipe Management
- Configuration Management
- Classification Management
- Workflow Management
- Change Management
- Portfolio Management
- Program Management
- Project Management
- Resource Management
- Quality Management (CAPA, Title 21 CFR Part 11 and Part 820, etc)
- Requirements Management
- Software Configuration Management
- Content Management
- Manufacturing Process Management
- Sourcing & Supply Chain Management
- Visualization & Markup
- Reporting & Analytics
- CAD, ERP and Office Tools Integration
- Ideation
- New Product Development (NPD) and Stage Gate Process
- Collaboration
- Architecture & Technology
- Automotive
- Aerospace & Defense
- Consumer & Consumer Packaged Goods
- Food & Beverage
- High-Tech & Electronics
- Industrial Machinery & Plant Equipment
- Medical Device & Life Sciences
- Process & Chemicals
This combination of leveraging industry best practice and company specific requirements distinguishes our PLM Requirements Specification and the results you can achieve significantly from working with and using other, more generic PLM RFP templates.
With our electronic PLM Requirements Specification you can:
- Quickly and methodically assess the suitability of specific PLM systems for your company’s needs
- Conveniently compare different systems
- Easily determine gaps between the required and the offered functionality
- Effortlessly create powerful and easy-to-understand management reports that include evaluation facts and results in every desired detail
Get your PLM selection right the first time. With 1,200+ proven requirements you can quickly define and select the best PLM solution for your business faster and with highest confidence. Get our PLM Requirements Specification and Software Analysis Tool now.